JTP supports excellent medical device technologies from overseas entering the Japanese market by providing one-stop services covering support for market entry to after-sales support
Japan’s medical device market is growing on the back of an increase in the senior citizen population and improved quality of medical care. The Japanese government positions the medical industry as one of the growth areas in the country, and the industry is expected to continue to see steady growth.
At JTP Co., Ltd.(JTP), we help facilitate overseas high tech companies entering the Japanese market with our expertise as well as contribute to international community by creating an open market in the world of international business and thereby raising the international status of Japan in the field of medical devices, in accordance with the corporate philosophy.
Since the launch of our medical device support business in 2003, we have been providing one-stop services ranging from product introduction and after-sales maintenance, inspection, repair, help desk, and all the way to device and supply management mainly for overseas medical device manufacturers for nearly 15 years.
In addition to these support services, JTP announced a provision of the Service package for Pharmaceuticals and Medical Devices Law in 2016. Through this service, which provides support for applying to obtain PMDL approvals when entering into a market and for selecting marketing channels, JTP provides even more comprehensive services covering market entry support to device support.
In this page, we will present the overall picture of medical device support services provided by JTP as well as the strength of its services and development going forward through interviews, etc. with customers who use our services.
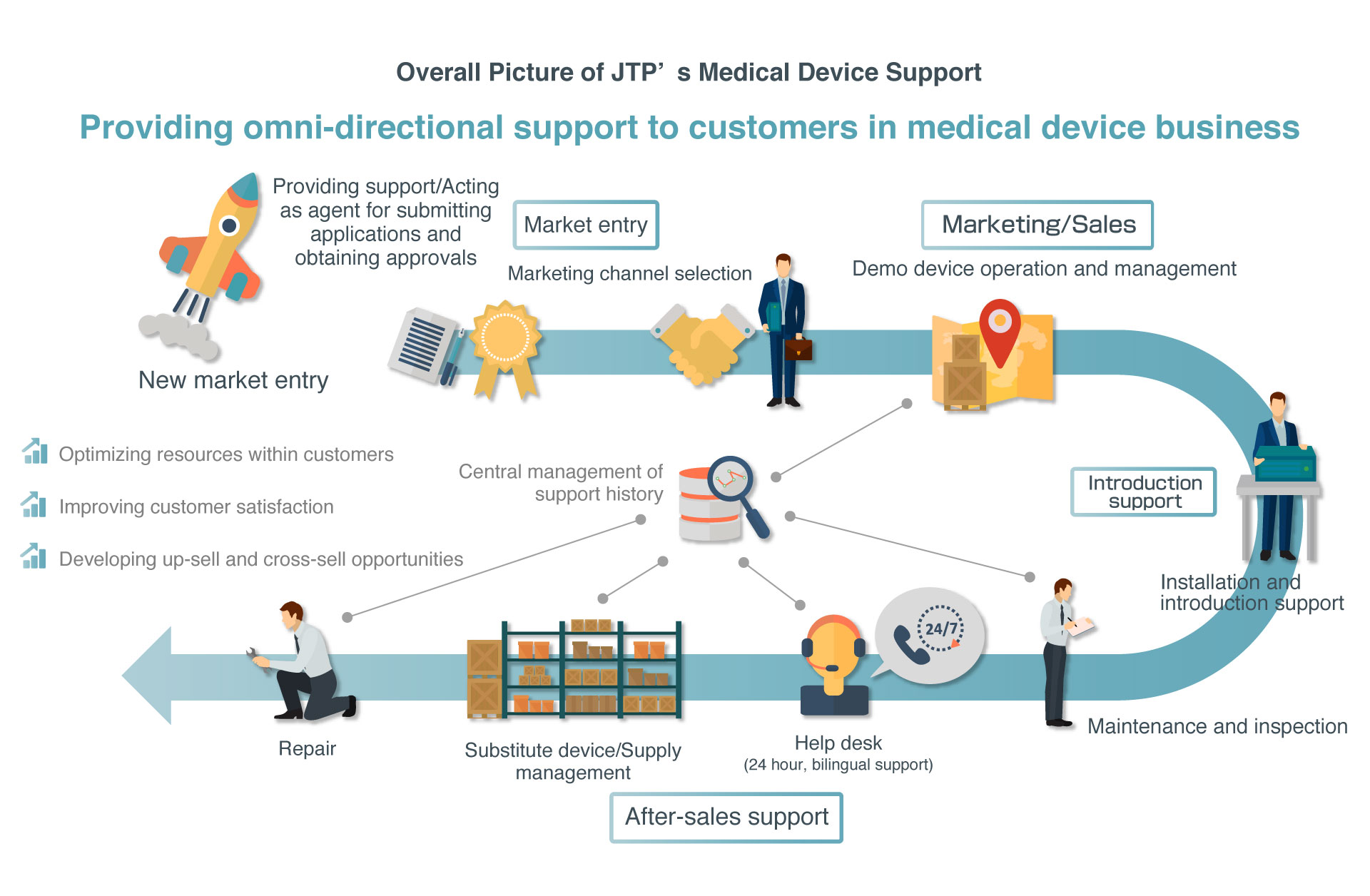
JTP provides a wide variety of support ranging from applying for PMDL approvals required for marketing medical devices in Japan as a proxy, new market entry support including selecting marketing channels, sales support through demo device operation and management, and after-sales support. As part of after-sales support, JTP provides one-stop services required for the medical device business, including not only introduction and installation support as well as repair, inspection, and other technical services, but also supply and substitute device management and a 24/7/365 help desk service.
JTP’s Strength in Medical Device Support

Providing Manufacturer-neutral Services
JTP provides services from a neutral position that is independent of specific manufacturers or devices, and has a track record of providing support for medical devices diverse fields.

Supporting the Use of More Advanced Information Technology
With its extensive track record of service in software solutions, JTP supports software and the advanced use of IT, in addition to providing hardware support.
Comprehensive Service
JTP can provide one-stop service covering applying for PMDL approvals required for marketing medical devices in Japan as a proxy, selecting marketing channels, and after-sales support.

Comprehensive Set of Licenses
JTP has licenses of Retail/Rental License of Specially Controlled Medical Devices and Medical Devices Repair License. JTP is able to offer appropriate services in compliance with the Japan Pharmaceuticals and Medical Devices Law (PMDL).
Customer Support in English
With bilingual engineers on staff, JTP offers device support and software training in English, also able to refer to original manual and able to communicate directly with engineers based overseas.
Tokyo Technical Lab Center
Service Facility with ISO13485 Compliant Quality Control System
JTP operates the Tokyo Technical Lab Center based on ISO13485; which is the International Organization for Standardization (ISO) standard for quality management system for medical devices, as the service base of its business for supporting medical devices. It is also the base of JTP’s teams of engineers and has various spaces for all kinds of tasks including help desk services. At the Tokyo Technical Lab Center, JTP’s teams closely collaborate to provide quality services.
Tokyo Technical Lab Center Overview
- Quality control system in compliance with the ISO 13485 international standard
- System of sending back repaired and inspected medical devices to customers
- Device and supply inventory management
- 24/7/365 help desk service

Client Story 1
Fujifilm Medical Co., Ltd.
Supported Medical Devices: Ultrasonic diagnostic equipment
Support Service Details: Demo device management, send-back repair, maintenance and inspection, help desk, supply and substitute (loaner) device management
The SonoSite series supplied by Fujifilm Medical Co., Ltd. is mobile ultrasonic diagnostic equipment, which has become popular in Japan recently due to its excellent portability and ability to allow the user to conduct inspection with fewer burdens on patients. The SonoSite series, which is compact and has high resolution images thanks to its image processing circuit enabled by Fujifilm Medical’s unique technology, is used for home healthcare, emergency treatments, and other wide-ranging medical care practices.
JTP has been providing special support services for the SonoSite series for a long time mainly at the Tokyo Technical Lab Center. The services are provided at the center, which include repair, inspection and other engineering services, a help desk service for end users, and inventory management for spare parts, supplies or substitute devices. JTP also supports for sales activities through management of demo devices.
In this interview, Kazuhiko Kitagawa, who has been engaged in building a partnership system between Fujifilm Medical and JTP as a sales representative, and Junko Nakayama, who leads collaboration between the two companies, mainly in logistics introduced about their business with JTP.

Kazuhiko Kitagawa
Manager in charge of Tokyo and South Kanto Region
Ultrasound Promotion Department Sales Div.
Fujifilm Medical Co., Ltd.

Junko Nakayama
Ultrasound Promotion Department Planning Div.
Fujifilm Medical Co., Ltd.
- Mr. Kitagawa
- The SonoSite series was launched in Japan by our small team consisting mainly of sales representatives, so we needed to outsource support services and back office tasks. Since it was a small startup, there were not sufficient internal resources and the company was cautious about investing in the business. Therefore it was extremely important to outsource specialized tasks. Today, since JTP has acquired various types of data through long years of support services, we have been continuing to outsource support services to JTP.
- Ms. Nakayama
- JTP has acquired various types of data in the course of providing support services for the SonoSite series.
This data serves not only as a record of the support provided, but also forms the basis for making decisions with regard to our in-house business operations. For example, when we purchase parts for a device, the repair history and management data on faulty locations or malfunctioning parts are both of great importance in determining the appropriate quantity of parts that it should carry in stock. Also, the utilization rate for demonstration equipment, the operation and maintenance of which is outsourced to JTP, serves as an important benchmark for sales.
- Mr. Kitagawa
- JTP’s help desk has collected feedback from customers. Based on such information, we can find new business opportunities through product upgrades, up-selling, or cross-selling. For this reason, JTP’s support service has come to serve as an important role in contributing to the success of our marketing activities, in addition to streamlining our business through the outsourcing of service tasks. We will continue to use data JTP has accumulated in the course of operations in a more strategic way.

Managing demonstration and substitute devices is extremely important from a perspective of marketing and improving customer loyalty.

At the Tokyo Technical Lab Center, JTP repairs devices used throughout Japan.
Client Story 2

Varian Medical Systems K.K.
Supported Medical Devices: Radiation therapy systems
Support Service Details: On-site technical support engineers for periodical inspections
JTP also provides technical support that specializes in on-site support services, and its technical support engineers conduct periodical inspections of Clinac-iX, a radiotherapy system provided by Varian Medical Systems K.K.
Due to the customers who use products of Varian Medical Systems having entered into maintenance agreements and individual support for each system through periodical inspections will lead to the stable operation of the system, Varian Medical Systems was facing an increase in the workload of the periodical inspection tasks. Today, Varian Medical Systems outsources part of its extensive inspection tasks to JTP and their engineers can now focus on more specialized tasks, such as repairing and adjusting the system’s main body.
Since Varian Medical Systems sells software products in addition to hardware, it will seek cooperation with JTP in software related operations as well going forward.
Comment from Mitchell Silong, Managing Director
Varian Medical Systems is striving to contribute to innovative and simplified cancer-fighting treatments by delivering radiation treatment technology and solutions under our mission “A World Without Fear of Cancer.”
Our company as the world leader in radio-oncology, designs, implements and supports radiotherapy solutions that enable medical institutions to deliver the latest cancer-fighting treatments. Currently, we are working with JTP Co., Ltd. (JTP) to install and support the Clinac-iXTM series of medical linear accelerators. JTP is a skilled partner and we look forward to continuing to grow together expanding the scope of collaboration.
Radiotherapy solutions require stringent quality control to deliver the prescribed treatment to exacting locations. The delivery of these treatments requires highly trained experts ensuring these strict quality standards are met. JTP provides these skilled resources and supports Varian’s solutions enabling Healthcare providers to deliver the high quality treatments to patients. We are very honored to work together with JTP.
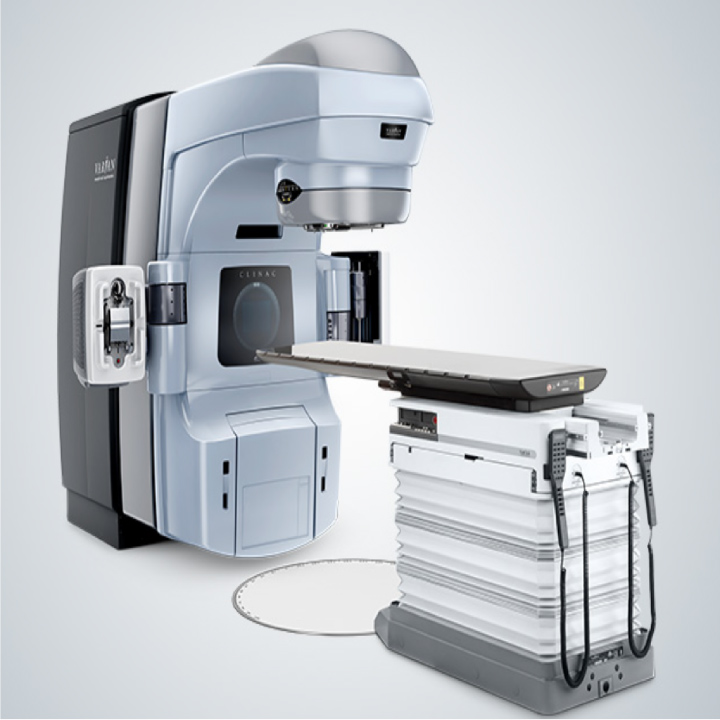 Clinac-iX
Clinac-iXMedical equipment approval number: 20400BZG00055000
Copyright ©2007,
Varian Medical Systems, Inc.
All rights reserved.
Medical Device Support: Development Going Forward
From medical device business support to the use of medical information technology
Based on our track record in the area of life sciences, JTP is committed to providing a comprehensive support service that better contributes to customers’ businesses.
As for the Service package for Pharmaceuticals and Medical Devices Law, which we started providing in June 2016, we now support not only overseas medical device manufacturers, who are our initially expected customers, but also other medical device manufacturers with excellent technologies in Japan in commercializing at a rapid pace.
In recent years, digitization of medical care is rapidly progressing. In addition to streamlining operations and optimizing information at medical institutions, such as the use of electronic medical records and hospital in-house network, more advanced technologies, such as artificial intelligence (AI), big data, and the Internet of Things (IoT), are proactively used in the field of medical devices.
It is said that medical information technology will create the database of a large volume of information accumulated through diagnosis and treatment practices, whereby achieving a society in which people can receive more appropriate and advanced medical treatment. In the United States, the use of medical big data, which uses genomics data, has already started as part of its national strategy, and cases of successful treatments have already been reported.
At JTP, outsourcing services in the area of medical devices and IT business support have been the major pillars of our business for many years. We have vast experience and expertise in the field of both IT and medicine, with a proven track record of installing hospital in-house networks and operating image servers.
Moreover, we have a significant advantage in the areas of advanced technology, including cloud computing, AI, robotics, and big data, which have become the focus of attention in recent years, as every one of our employees has been constantly striving to acquire the latest technology. With such IT skills and expertise regarding medical device support, we aim to strengthen our business in the medical IT field, which is expected to see further growth, and thereby providing support for improving the medical care environment in Japan.

Yutaka Mori
President and CEO
Related Information
Contact Us/Request Information
Contact Us/Request InformationClick here for inquiries on Life Science





