Service package for Pharmaceuticals and Medical Devices Law
JTP provides total solution for entering medical device market in Japan, from the approval process to the post-marketing support.
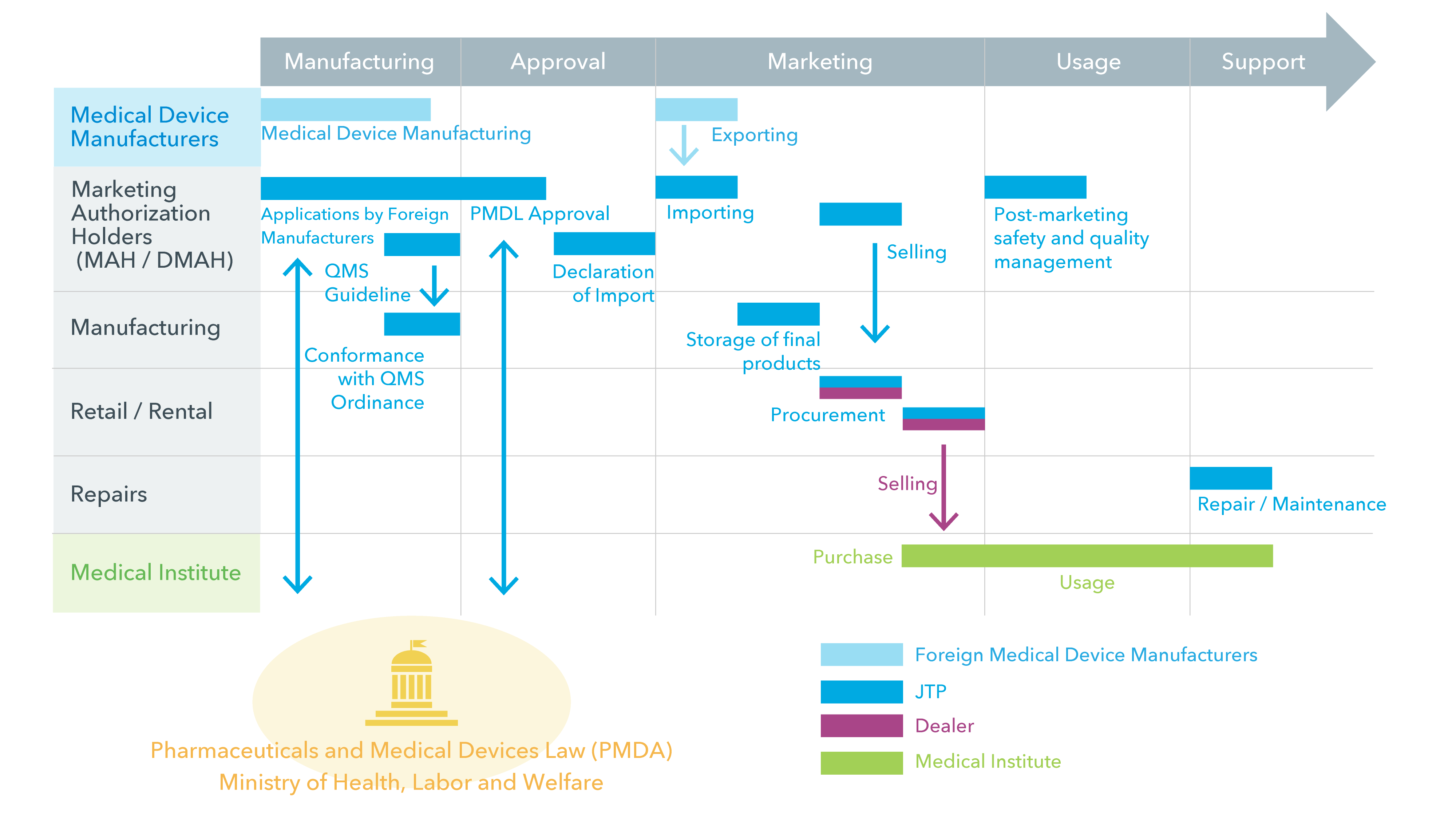
The medical devices market in Japan is growing continuously by the rapid aging in Japan. In 2014, the medical device regulations has revised to Pharmaceuticals and Medical Devices Law (PMDL) from the Japan’s Pharmaceutical Affairs Law (PAL). Since medical devices classification has added to PMDL, the regulation is optimized for medical devices which selling in shorter cycle compared from pharmaceuticals. The license system and classification had been simplified and mitigated, so it makes easier to enter the market in Japan. But still, the complicated processes of approval and classification of the devices are different from foreign market, and it is a challenge for the manufacturer. JTP provide continuous support for entering the market to business development by offering the total support including acting for approval, marketing channel selection and post-marketing support.
Market Entry Challenges for Overseas Manufacturers
Japan-Specific Device Classification and the Application Process
Market-entry to Japan’s market got to be a little bit simpler by revising from PAL to PMDL. But still, holding the certain licenses and meeting the Japanese law are required to sell the medical devices in Japan.
Expanding Sales Channels
In Japan, medical device manufacturers do not sell directly their own products to medical institute and go through a medical device dealer to do so. This means that in order to enter the market, foreign manufacturers need to make relationships with medical device dealers.
Operations in Japanese language
All documentation and applications are required to be prepared in Japanese, and communication with all related entities including the dealers need to take place in the local language.
Our Strengths
Comprehensive Set of Licenses
JTP holds licenses of Manufacturer, Marketing Authorization Holders, Retail/Rental License of Specially Controlled Medical Devices and Medical Devices Repair License. JTP is able to offer a full range of services in compliance with the Japan Pharmaceuticals and Medical Devices Law (PMDL).
Extensive Experienced Throughout Japan
JTP has many years of experience throughout Japan in providing support services for medical devices. The services are including installation support, repairs and maintenance, as well as offering a 24/7/365 help desk service.
Connections with Nationwide Dealers
JTP has connections with medical devices dealers from supporting experiences. The connections over 300 dealers nationwide makes developing sales channels for market entry to the manufacturers.
IT Expertise
JTP has many years of experience in the IT outsourcing industry, enabling them to offer high-quality IT support services in such as network construction, big data solutions, cloud computing, and security.
Service Offering Includes Post-Marketing Services
JTP handles all processes to sell the products to medical institutes on behalf of the manufacturers. The one-stop support solution reaches the approval process, the sales channel select and the post-marketing support (repair/check). This service makes your business cost effective by fast and suitable entering to the market in Japan.
References
Medical Devices Approval Process in Japan
When medical devices enter the Japan market, a regulatory application is required in accordance with the Pharmaceuticals and Medical Devices Law. This is an overview of the various requirements and process involved.
Read More…
Download materials on the classifications of medical devices and PMDL revision in Japan
These materials provide a following contents to know the details about medical devices in Japan:
– summary of revisions to the Japan Pharmaceutical and Medical Device Law (PMDL)
– information on the classifications related with medical devices, licenses, approval and repairs
Contact Us/Request Information
Contact Us/Request InformationClick here for inquiries on Service package for Pharmaceuticals and Medical Devices Law
