Service package for PMDL
Medical Devices Approval Process in Japan
Devices are required to undergo regulatory approval based on the Pharmaceuticals and Medical Devices Law (PMDL) in order to enter the Japan market. The law, revised in 2014, includes new provisions for medical devices, which had been treated the same as pharmaceutical products prior to the revision.
Types of Licenses and Application Processes
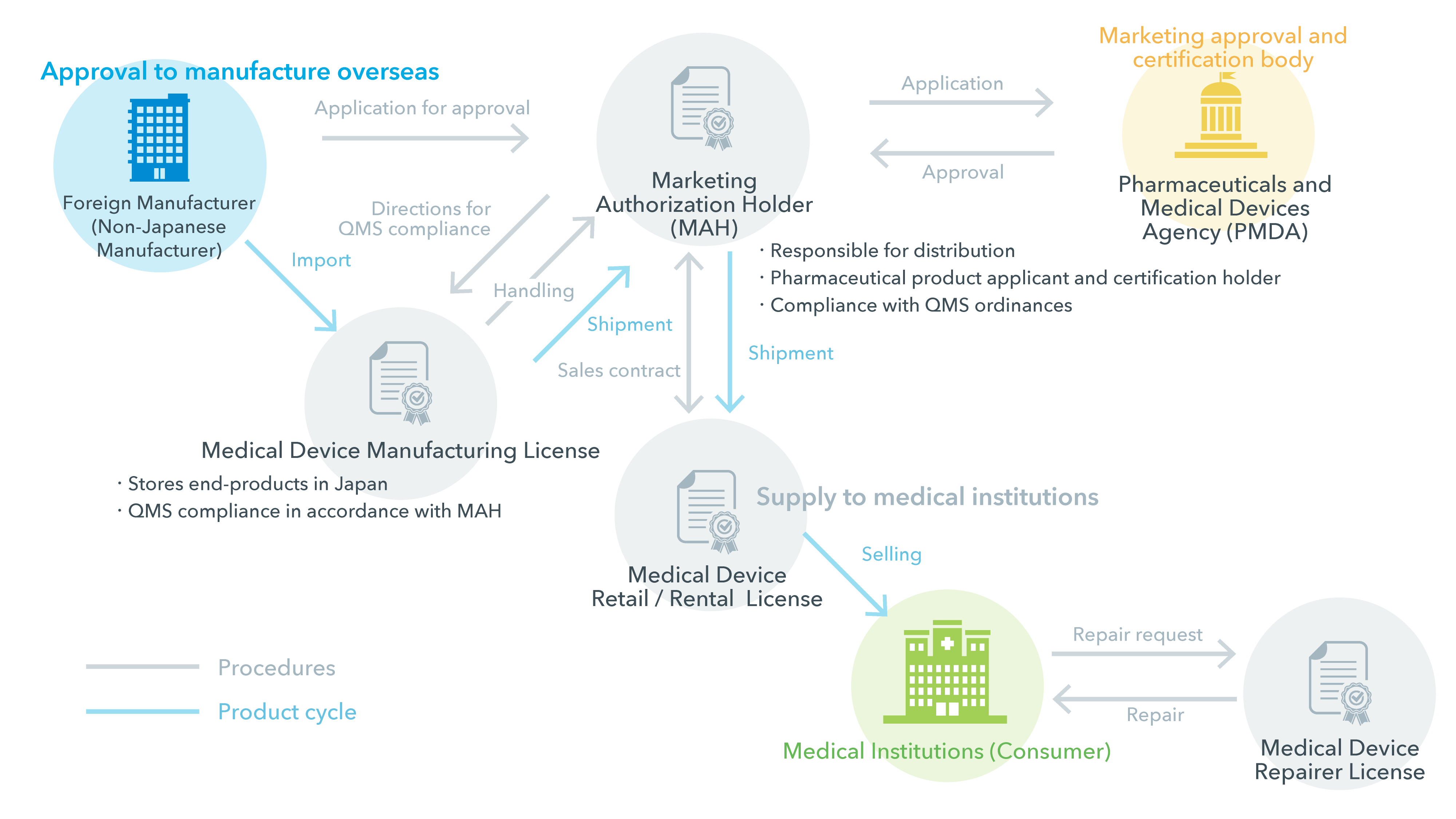
| Manufacturing License | A manufacturing license not only permits the design and manufacturing of medical devices, but also the sterilization of devices, storage of end-products in market (this includes packaging, labeling, and managing package inserts). Manufacturers are required to comply with standards stipulated in the QMS Ordinance that presents quality control guidelines for manufacturing and sales operations. Manufacturers are not permitted to sell medical devices directly to medical institutions, and can only market and ship devices to Marketing Authorization Holders. |
|---|---|
| Marketing Authorization Holders License
(MAH / DMAH) |
Throughout the medical device import-to-market process, MAH handle regulatory applications such as import permissions and pharmaceutical applications, instruct on and comply with regulatory ordinances, as well as manage sales channels. The scope of their duties is also determined by the classification of the medical devices. As manufacturers are not permitted to market directly to medical institutions, they sell the medical devices to MAH and retail/rental license holders. |
| Retail / Rental Service License | Organizations with retail and rental service licenses can sell medical devices to medical institutions or other retail and rental service license holders. Depending on the medical device classification, the organization may also need to acquire certification for highly advanced controlled medical devices or the status of notification for controlled medical devices. |
| Repairs License | This license has two categories that determine the scope of repairs; Specially controlled and Non-controlled. Each of these categories has 9 product classifications, and organizations are required to hold the necessary licenses for the specific classification of the product handled. |
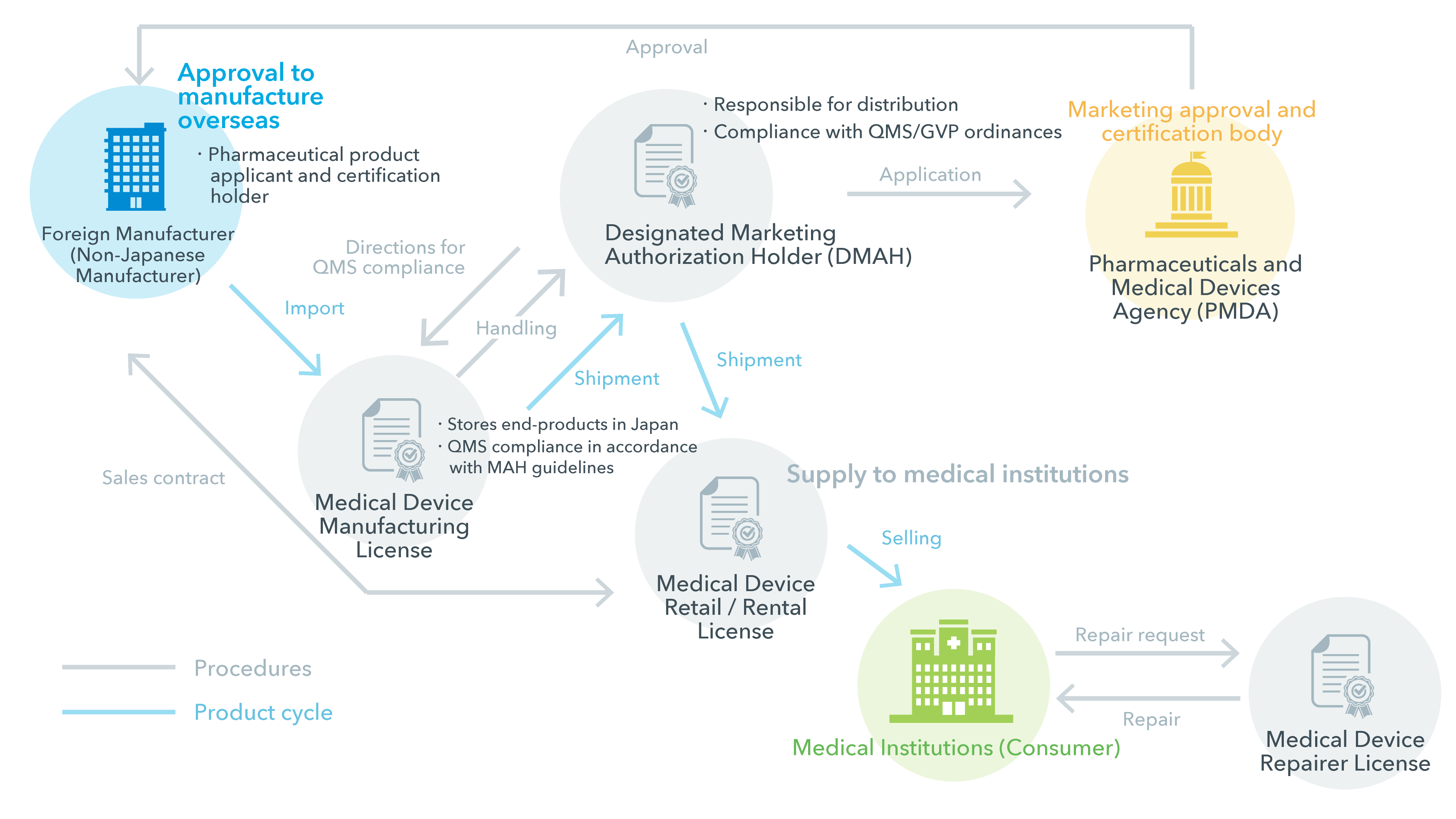
Marketing Authorization Holder (MAH) and Designated Marketing Authorization Holder (DMAH)
In order to sell products in Japan, non-Japanese manufacturers first need to be approved as certified Non-Japanese Manufacturers. Then, they must appoint a Marketing Authorization Holder (MAH) to apply to register their products with the Pharmaceuticals and Medical Devices Agency (PMDA) in order to get permission to market their product in Japan. There are two types of representatives that can do this; the Marketing Authorization Holder (MAH) and the Designated Marketing Authorization Holder (DMAH).
In the case of a Marketing Authorization Holder, the application is carried out by the MAH in its name and approval is granted to the MAH.
In the case of the DMAH, the DMAH handles the application on behalf of the foreign manufacturer, and approval itself is granted to the foreign manufacturer. This is referred to as foreign exceptional approval. The application approval would be owned by the manufacturer, which then does not need to rely on a marketing authorization holder.
Each of the license holders supports end-users through the required procedures of the manufacturing to post-marketing process. MAH is responsible for most of the process, handling everything from the pre-marketing applications to post-marketing safety and quality control management.
Pre-Marketing Applications to Post-Marketing Services
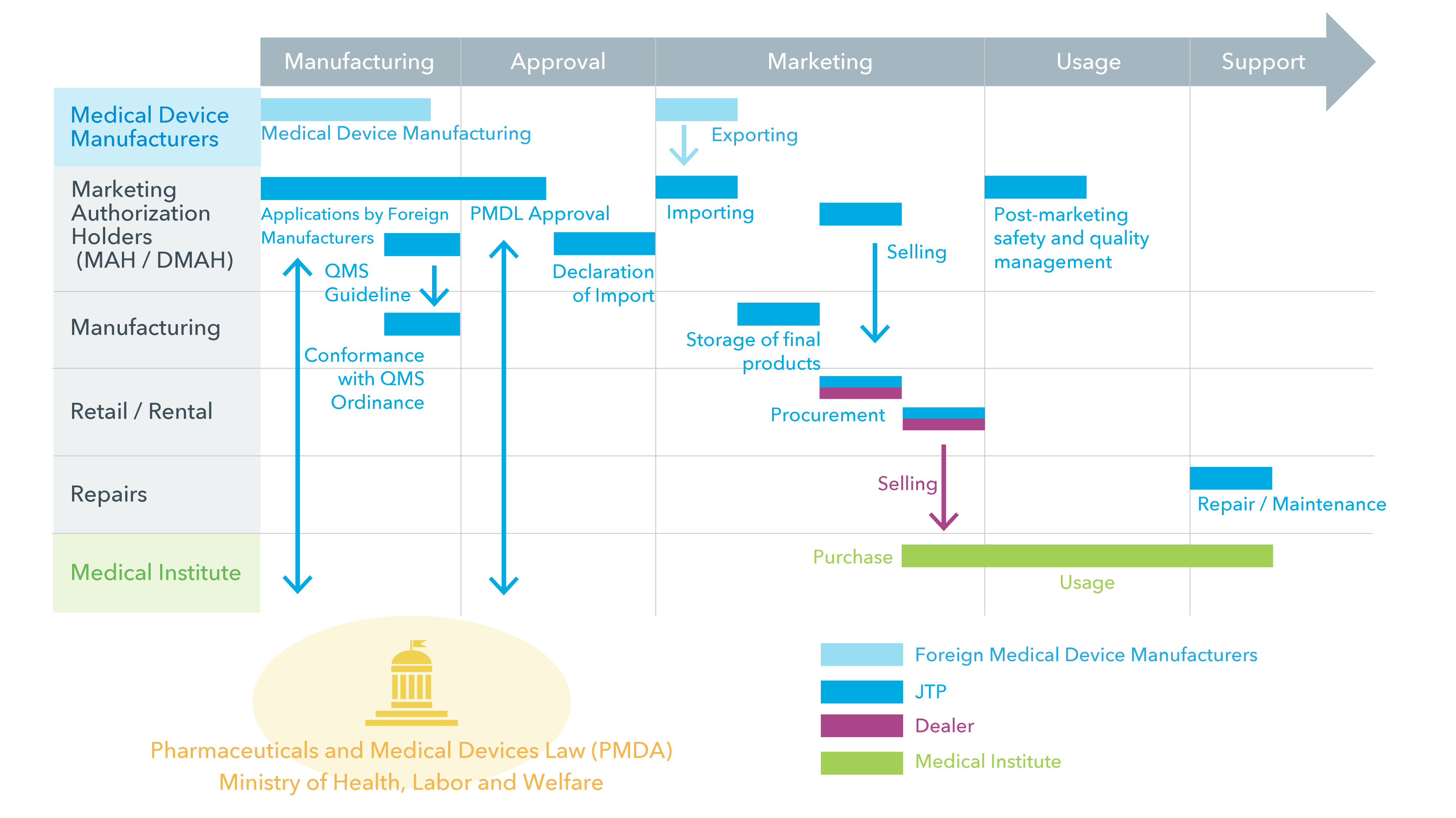
Related Organizations and Applications
| Ministry of Health, Labour and Welfare (MHLW) | This administrative body is responsible for overseeing all matters concerning healthcare, welfare and labor policy in Japan. The Minister of Health, Labor and Welfare is also responsible for signing off on pharmaceutical product applications. However, the actual application process is carried out by the Pharmaceuticals and Medical Devices Agency (PMDA), which falls under the jurisdiction of the MHLW.
|
|---|---|
| The Pharmaceuticals and Medical Devices Agency (PMDA) | The PMDA has three key responsibilities of reviewing applications, ensuring safety, and compensation in relation to drug products and medical devices. It handles the review of market authorization applications based on Japanese pharmaceutical law, inspection duties, and the provision of information. Marketing Authorization Holders submit applications to the PMDA for approval to market medical devices in Japan.
|
| Pharmaceuticals and Medical Devices Law (PMDL) | The Law for Ensuring the Quality, Efficacy, and Safety of Drugs and Medical Devices (The Pharmaceutical and Medical Devices Law/PMDL) is a law regulating the manufacturing and marketing of pharmaceutical products and medical devices. Before the PMDL was revised it was referred to as the Pharmaceutical Affairs Law (PAL). The 2014 revisions included items that factor in characteristics of medical devices and the relaxation of regulations concerning the manufacturing and marketing of medical devices, enabling a faster import-to-market passage. Also, the revised law includes regenerative medicine such as iPS cell and embryonic stem cells (ES cells). |
| QMS Ordinance | The QMS (Quality Management System) for medical devices is a set of standards for manufacturing control and quality control, based on ISO 13485. Marketing Authorization Holders serve in a key role in directing manufacturers on how to comply with these standards, based on the QMS ordinance. |
| GVP Ordinance | The GVP (Good Vigilance Practice) Ordinance stipulates a set of safety control standards for the manufacturing and marketing of medical devices, and post-marketing compliance with these standards is also required. |
Download materials on the classifications of medical devices and PMDL revision in Japan
These materials provide a following contents to know the details about medical devices in Japan:
– summary of revisions to the Japan Pharmaceutical and Medical Device Law (PMDL)
– information on the classifications related with medical devices, licenses, approval and repairs
Contact Us/Request Information
Contact Us/Request InformationClick here for inquiries on Service package for Pharmaceuticals and Medical Devices Law