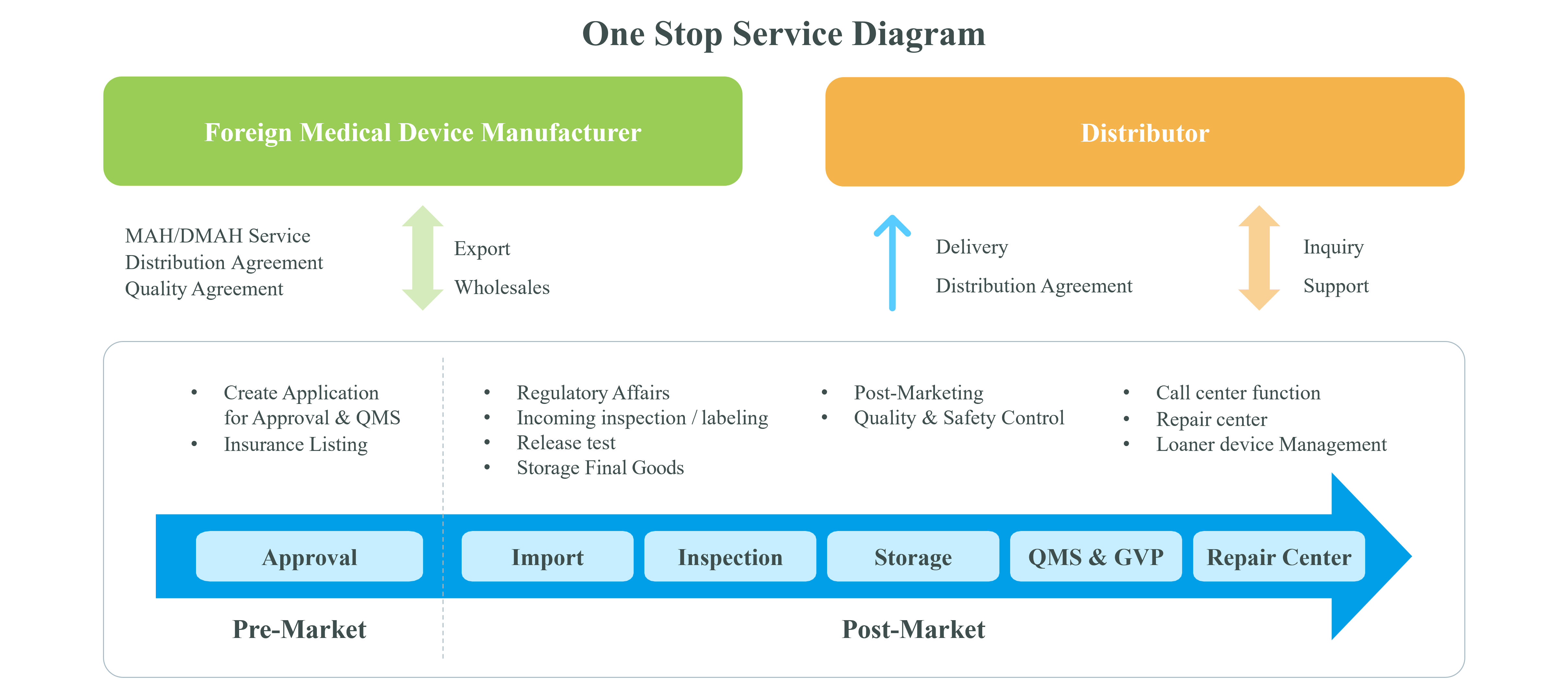
JTP provides total solution for entering medical device market in Japan, from the approval process to the post-marketing support.
Use Case
Market Entry Challenges for Overseas Manufacturers
Our Strengths
Service Offering Includes Post-Marketing Services
JTP handles all processes to sell the products to medical institutes on behalf of the manufacturers. The one-stop support solution reaches the approval process, the sales channel select and the post-marketing support (repair/check). This service makes your business cost effective by fast and suitable entering to the market in Japan.
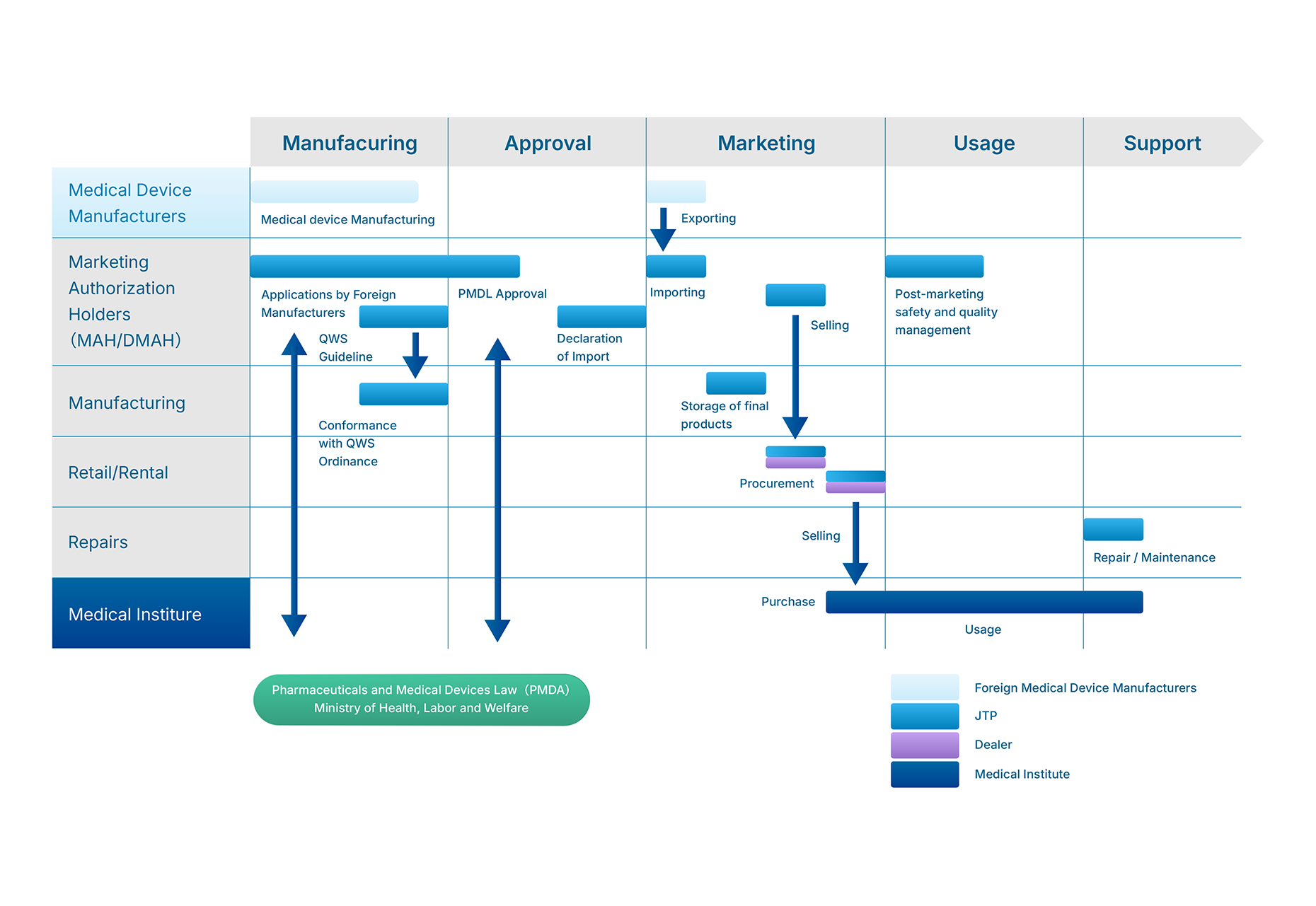

Download materials on the classifications of medical devices and PMDL revision in Japan
These materials provide a following contents to know the details about medical devices in Japan:
– summary of revisions to the Japan Pharmaceutical and Medical Device Law (PMDL)
– information on the classifications related with medical devices, licenses, approval and repairs

