JTP supports excellent medical device technologies from overseas entering the Japanese market by providing one-stop services covering support for market entry to after-sales support
Japan’s medical device market is growing on the back of an increase in the senior citizen population and improved quality of medical care. The Japanese government positions the medical industry as one of the growth areas in the country, and the industry is expected to continue to see steady growth.
At JTP Co., Ltd.(JTP), we help facilitate overseas high tech companies entering the Japanese market with our expertise as well as contribute to international community by creating an open market in the world of international business and thereby raising the international status of Japan in the field of medical devices, in accordance with the corporate philosophy.Since the launch of our medical device support business in 2003, we have been providing one-stop services ranging from product introduction and after-sales maintenance, inspection, repair, help desk, and all the way to device and supply management mainly for overseas medical device manufacturers for nearly 15 years.In addition to these support services, JTP announced a provision of the Service package for Pharmaceuticals and Medical Devices Law in 2016. Through this service, which provides support for applying to obtain PMDL approvals when entering into a market and for selecting marketing channels, JTP provides even more comprehensive services covering market entry support to device support.
In this page, we will present the overall picture of medical device support services provided by JTP as well as the strength of its services and development going forward through interviews, etc. with customers who use our services.
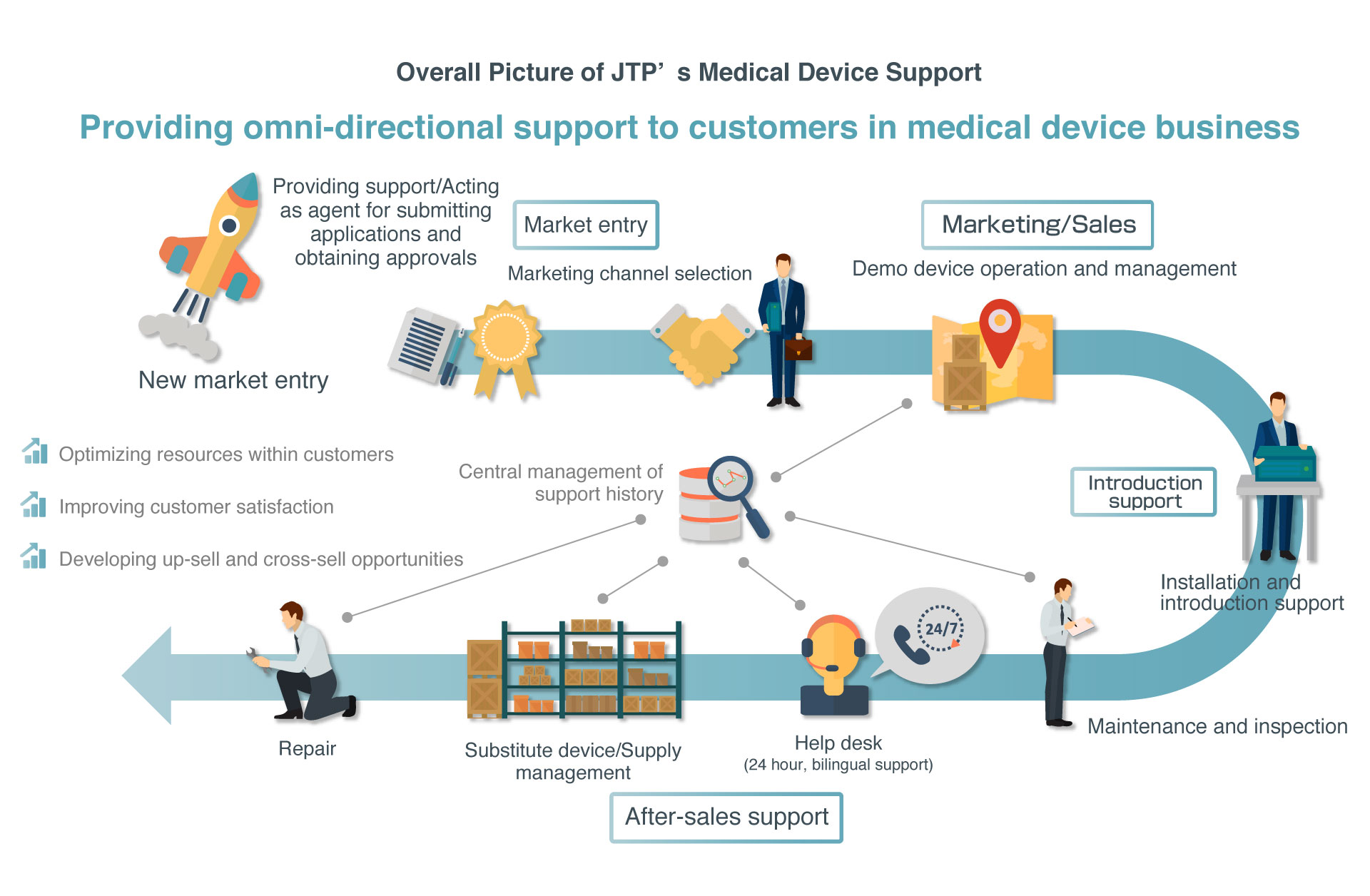
JTP provides a wide variety of support ranging from applying for PMDL approvals required for marketing medical devices in Japan as a proxy, new market entry support including selecting marketing channels, sales support through demo device operation and management, and after-sales support. As part of after-sales support, JTP provides one-stop services required for the medical device business, including not only introduction and installation support as well as repair, inspection, and other technical services, but also supply and substitute device management and a 24/7/365 help desk service.
JTP’s Strength in Medical Device Support
Tokyo Technical Lab Center
Service Facility with ISO13485 Compliant Quality Control System
JTP operates the Tokyo Technical Lab Center based on ISO13485; which is the International Organization for Standardization (ISO) standard for quality management system for medical devices, as the service base of its business for supporting medical devices. It is also the base of JTP’s teams of engineers and has various spaces for all kinds of tasks including help desk services. At the Tokyo Technical Lab Center, JTP’s teams closely collaborate to provide quality services.
Tokyo Technical Lab Center Overview
- Quality control system in compliance with the ISO 13485 international standard
- System of sending back repaired and inspected medical devices to customers
- Device and supply inventory management
- 24/7/365 help desk service









